What is eyonis®
eyonis® is a suite of Software as a Medical Device (SaMD) that leverages advanced Data Science (DS) & Artificial Intelligence (AI) technologies to analyze medical imaging, supporting radiologists & healthcare professionals in identifying cancers at the earliest stage, even when invisible to the human eye.
Our Mission
Shifting the imaging diagnostic paradigm by designing innovative AI/ML tech-based SaMDs and supporting radiologists & healthcare professionals worldwide in screening, early diagnosis & treatment of cancer.
Our Vision
Establishing eyonis® as a Standard-of-Care (SoC) to reduce cancer burden by helping radiologists & healthcare professionals to identify disease at the earliest stage, allowing better care while avoiding unnecessary medical procedures & reducing healthcare costs.
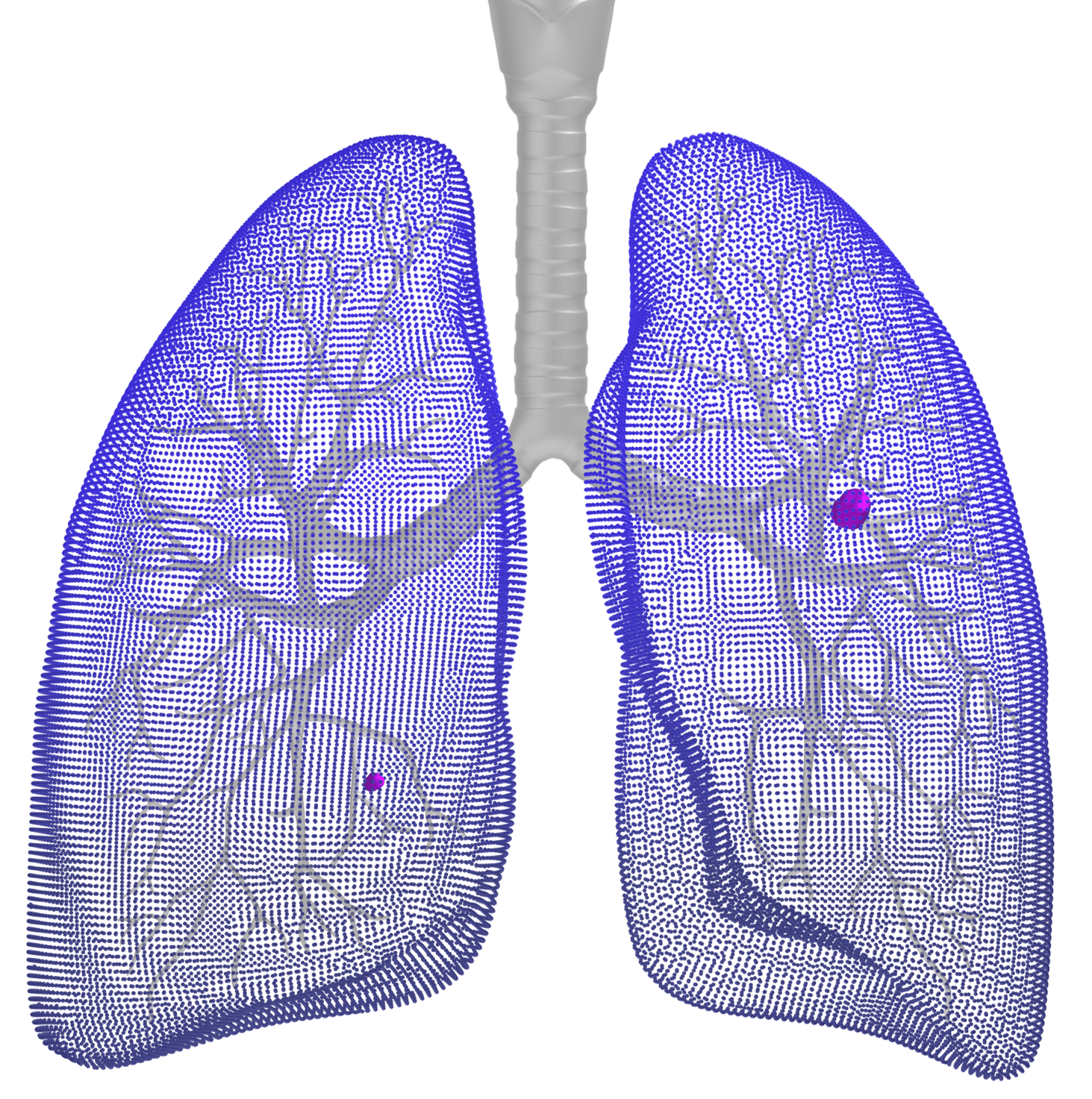

Software as a Medical Device for Lung Cancer Screening (LCS)
eyonis® LCS is an AI/ML technology-based end-to-end CADe/CADx Software as a Medical Device (SaMD) developed to aid cancer diagnosis and improve clinical management of patients, designed to:
enable early detection, localization & diagnosis of solid & part solid pulmonary parenchymal nodules*, 4-30 mm Longest Average Diameter (LAD) on Chest Low-Dose Computed Tomography (LDCT) images
generate DICOM file result report, PACS available & compatible with every radiologist’s viewer
list all probably benign, suspicious, very suspicious, lung nodules present in LDCT image series
score nodules individually by giving a malignancy score & providing a Likelihood of Malignancy (LOM%) from 1% to 93%.
eyonis® LCS processing chain
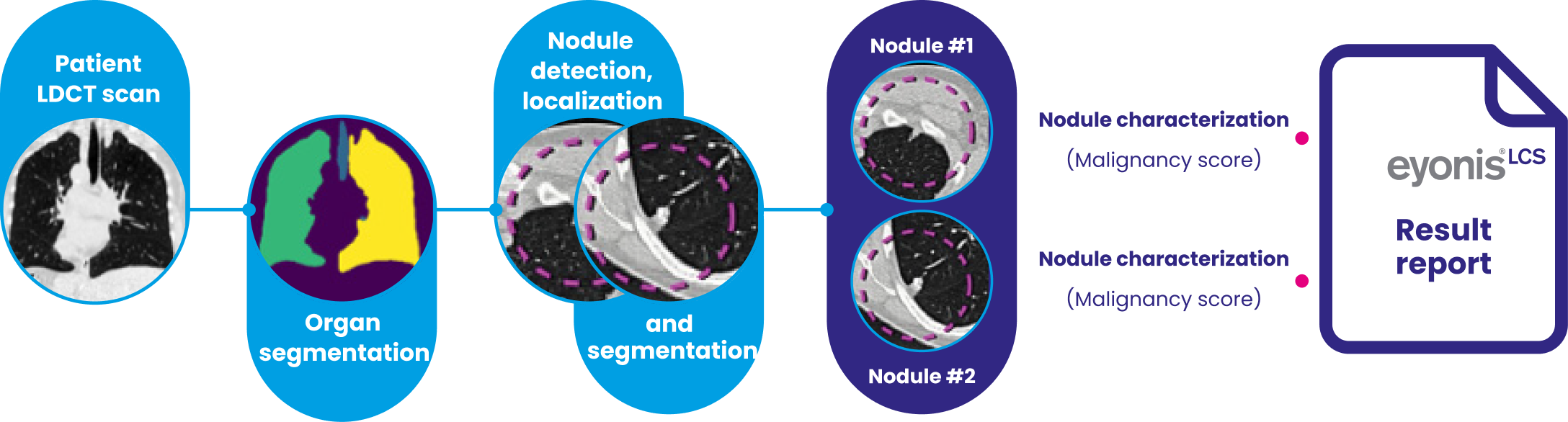
eyonis® LCS contains methods protected by patents granted in the U.S and pending in other countries
PATENT US 11 810 299 “Method and system for computer aided diagnosis using ensembled 2D and 3D neural networks based on medical images”
PATENT US 12 272 063 “Apparatuses and methods for training and using computational operations for digital image processing”
For each nodule detected eyonis® LCS result report provides
Slice number
(feet to head & head to feet)
Malignancy Score from 1 to 10
(color-blind friendly)
Diameters (Long/Short/Average) & Volume
Full slice snapshot
containing nodule
Close-up snapshot
of nodule
Robust AI algorithm trained, tested and validated on independent enriched U.S. and EU cohorts, achieving performance above the current state of the art
Median Technologies, data on file
AUC: Area Under the Curve is a discrimination measure which tells us how well we can classify patients in two groups: those with and those without the outcome of interest
With eyonis® LCS, our innovative CADe/CADx SaMD, we empower healthcare professionals to reduce both false positives & false negatives, standardize diagnosis, and save time and costs
of cancer cases identified (sensitivity)
of benign cases identified (specificity)
False Negative reduction*
False Positive reduction*
of Negative Predictive Value (NPV)
* Estimated from Jonas DE, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021 Mar 9;325(10):971-987.
Flexible & seamless deployment
Cloud-based DICOM images are collected via the eyonis® Gateway* & sent to the eyonis® Cloud for processing. The final result report is then returned to the Institution
Local hosting eyonis® LCS & eyonis® Gateway are locally installed in the Institution
*eyonis® Gateway enables communication between PACS & eyonis® product to ensure image transport
Posters
- Radiologists perception on AI/ML Software as a Medical Device (SaMD) unveiled via post-study usability survey: key assets to redefine Lung Cancer Screening practice – Presented at the ESMO AI & Digital Oncology Congress in Germany – November 2025
- AI-assisted Lung Cancer Screening: Results from REALITY, a pivotal validation study of an AI/ML-based software – Poster presented at the American Thoracic Society Congress in San Francisco – May 2025
- Budget impact model of enhanced Lung Cancer Screening with AI/ML tech-based software as a medical device (SaMD) on a us cohort and private payer perspective – Poster presented at ISPOR US in Montréal – May 2025
- AI/ML-based lung cancer detection and characterization for lung cancer screening: results from the REALITY study on early-stage lung cancer – Presented at European Lung Cancer Congress in Paris – March 2025
Certifications and partnerships
ISO 27001

HDS (Hébergement de Données de Santé – Health Data Hosting)

IHE Connectathon Seal
During the 2025 Connectathon, Median eyonis® team successfully validated eyonis® LCS interoperability and data consistency.
Four IHE profiles as Evidence Creator
One IHE profile as Time Client





Four IHE profiles as Evidence Creator




One IHE profile as Time Client

